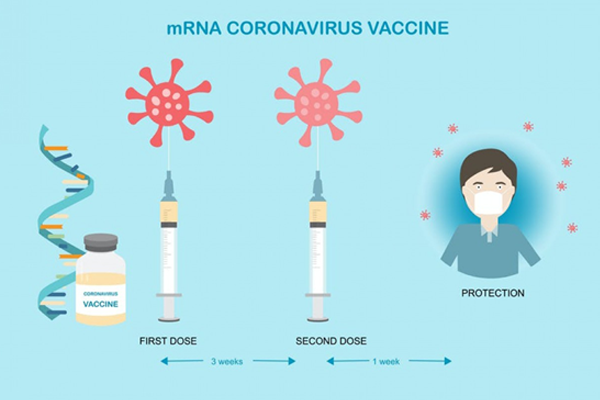
 Pune-based biotechnology company, Gennova Biopharmaceuticals Ltd (GBL), has developed the country’s first mRNA (messenger ribonucleic acid) based Covid-19 vaccine. It had submitted the interim clinical data of the Phase-I study to the Central Drugs Standard Control Organisation (CDSCO), which is the union government’s National Regulatory Authority (NRA).Vaccine Subject Expert Committee (SEC) has reviewed the interim phase-I data and concluded that HGCO19 (Covid-19 vaccine) has been found to be tolerable, safe, and immunogenic on the participants on whom its efficacy was tested.
Pune-based biotechnology company, Gennova Biopharmaceuticals Ltd (GBL), has developed the country’s first mRNA (messenger ribonucleic acid) based Covid-19 vaccine. It had submitted the interim clinical data of the Phase-I study to the Central Drugs Standard Control Organisation (CDSCO), which is the union government’s National Regulatory Authority (NRA).Vaccine Subject Expert Committee (SEC) has reviewed the interim phase-I data and concluded that HGCO19 (Covid-19 vaccine) has been found to be tolerable, safe, and immunogenic on the participants on whom its efficacy was tested.
 GBL has submitted phase-II and phase-III study for the purpose of evaluation of safety, tolerability, and immunogenicity of the HGCO19 in healthy participants. The efficacy would be tested in India approximately between 10-15 sites in phase-II and between 22-27 sites in phase-III.
GBL has submitted phase-II and phase-III study for the purpose of evaluation of safety, tolerability, and immunogenicity of the HGCO19 in healthy participants. The efficacy would be tested in India approximately between 10-15 sites in phase-II and between 22-27 sites in phase-III.
Gennova’s mRNA-based corona virus vaccine development programme was partly funded by the Department of Biotechnology (DBT). Later, it got further support from DBT under the Mission Covid Suraksha- The Indian Covid-19 Vaccine Development Mission, implemented by BIRAC.
The success of phase 1 study is a significant milestone for India and positions it on the Global Map for Covid-19 Vaccine Development. GBL is investing in increasing its manufacturing capacity to cater to the country’s vaccine requirements.